Wearable Technology Transforms Diabetes Care
 By Roger Bohannan | September 24, 2018
By Roger Bohannan | September 24, 2018
The World Health Organization predicts that chronic disease prevalence will rise by 57% by the year 2020, and that 10% of the world’s population will suffer from diabetes by 2045. At the same time, healthcare systems around the world are dealing with funding challenges, as governments face increasing pressure to reduce costs while a growing shortage of human resources is exacerbating the situation.
Consequently, with healthcare reform high on the agenda, collaboration with the private sector is increasing as governments seek low-cost, efficient solutions without impacting quality or access to care.
Technology has a significant role to play in the provision of healthcare, with advanced data and analytics enabling transformation of healthcare delivery models.
With healthcare resources under pressure, the ratio of practitioners to patients is inevitably dropping, with a consequent impact on patient access to care. This situation is more acute in rural areas, where there is less access to specialists and people must travel farther for treatment. More than 30% of diabetes patients, including all who suffer from type 1 diabetes, self-administer a prescribed level of insulin on a daily basis. However, healthcare practitioners may only have access to a patient’s average blood glucose levels four times per year, meaning that prescribed insulin dosages may not match the medical needs of the patient – particularly when blood glucose levels are fluctuating throughout the day, based on food intake, exertion levels, etc. Such mis-matches can lead to hospitalizations for hypoglycemia or diabetic ketoacidosis, a life-threatening condition.
This is one specific area of healthcare where connected medical technology is making a difference, with the growth in adoption of cloud-connected glucose meters and continuous glucose monitoring (CGM) devices. In recent years a number of these devices have appeared on the market.
CGM devices automatically transmit glucose readings from wearable devices to smartphone apps or cloud-based platforms, enabling patients to generate more comprehensive data on their specific illness, which doctors can then use to make more accurate diagnoses.
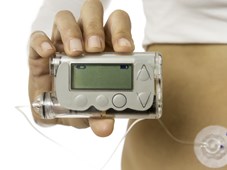
Insulin pump therapy, another development enabled by wearable, connected technology, eliminates the need for multiple daily insulin injections. Insulin pumps are small and lightweight (typically measuring about 5.7 × 7.6 × 1.9cm and weighing between 57 and 113g), and are easily concealed in clothing and attached to the body via a thin tube or infusion set. These pumps provide a constant background infusion of insulin that can be adjusted according to individual need, compensating for daily activity and exercise routines, and can also be programmed to deliver bolus doses of insulin to address the big glucose swings in the blood that would otherwise result from eating and drinking.
The design and manufacture of these devices is strictly regulated by the relevant authorities, such as the U.S. Food and Drug Administration (FDA), and they must comply with precise design and construction processes, meeting stringent documentation, development testing, production testing and field maintenance requirements. They are also required to incorporate comprehensive self-test and fault-indication capabilities, requiring the use of components that include self-test features. Since regulatory approval is a lengthy and costly process, manufacturers need to be confident that the chosen system components are not just highly reliable, but will be available for many years. Choice of and confidence in suppliers is therefore critically important for manufacturers of these devices, applying to every component, including the switches used to control the devices.

By Roger Bohannan, Medical Segment Leader, C&K
